We just found out that our R03 grant application for high-resolution BOLD fMRI at 7T has been recommended for funding. We have been anxiously waiting for the funding decision which was delayed by the government shutdown last year. Better late than never!
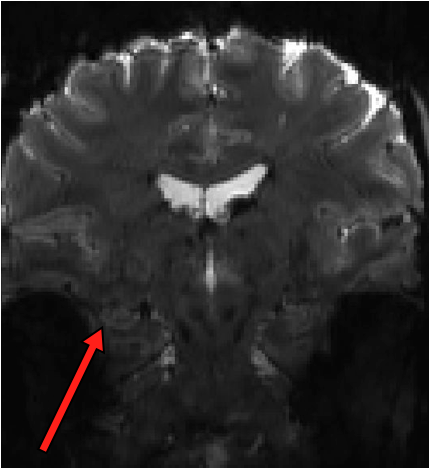
This pilot project will allow us to figure out the best way to achieve high resolution (1×1 mm in-plane) EPI in the medial temporal lobe without sacrificing too much SNR. The challenges here are manifold. Small voxels are SNR-starved, particularly in the MTL, and 7T introduces a boatload of spatial distortion. We have been acquiring some techdev scans over the past year and a half (see an example of raw EPI data below; arrow points to hippocampus showing how the tissue layers are resolved), and we are cautiously optimistic that acceptable signal quality can be achieved. Now we can go full throttle to systematically test various acquisition and analysis strategies to get the best possible data.
Functional connectivity within MTL
There is a ton of work looking at BOLD functional connectivity (FC) in Alzheimer’s Disease, and one consensus that has emerged is that this is a disconnection syndrome, with most studies finding weaker connectivity within the default mode network (DMN), including connectivity to DMN cortex from the hippocampus and/or MTL. But what happens inside the MTL ? There is intricate feedback circuitry within the MTL, highlighted by the perforant path that connects extra-hippocampal cortex to the hippocampus. Thanks to our automated MTL subregion labeling technique (implemented in the publicly available ASHS software), we are in a position to carefully label MTL subregions and measure connectivity between these. That’s what we did in a paper published in Hippocampus last year. This work was in collaboration with David Wolk at the Penn Memory Center.
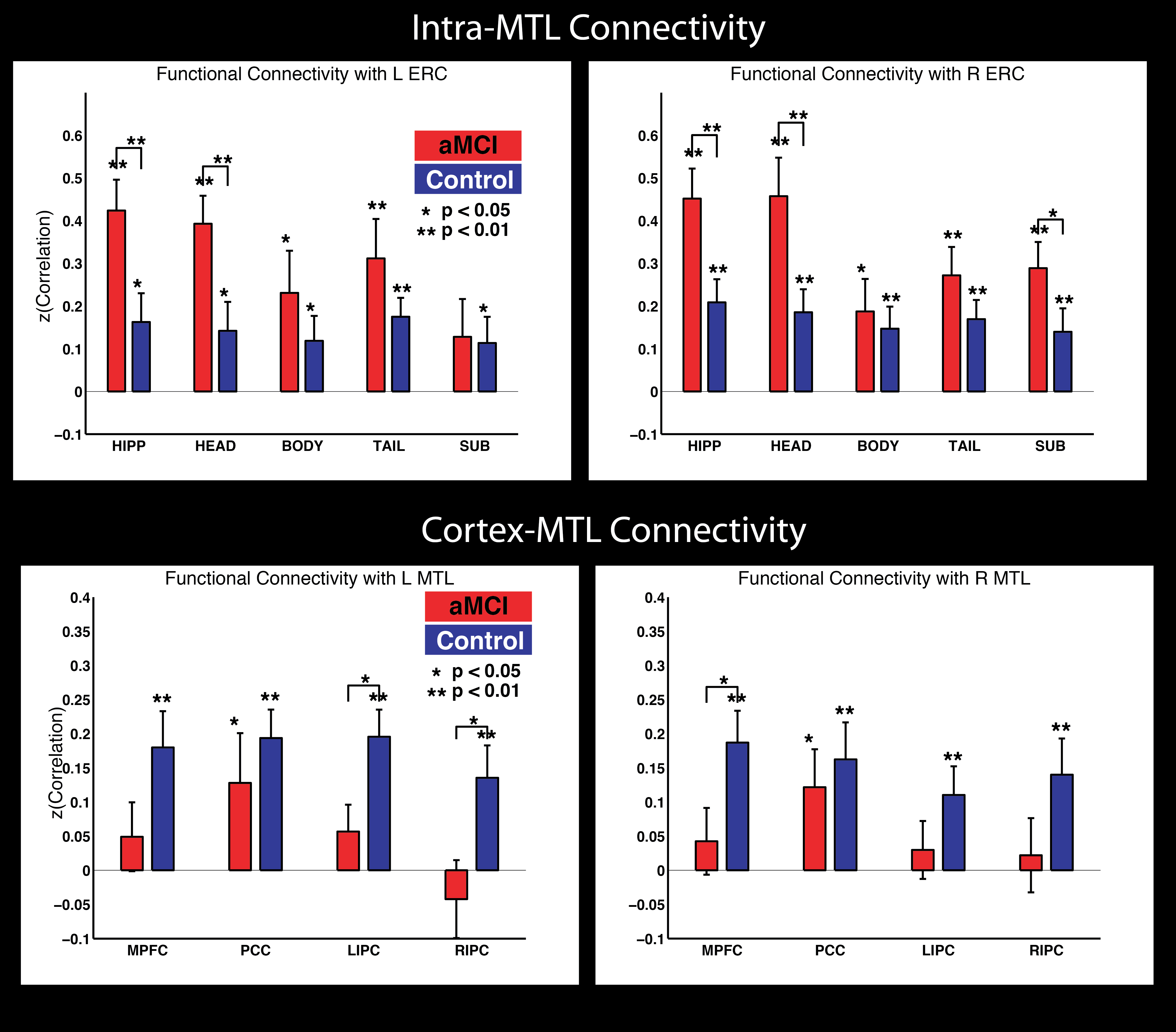
This figure summarizes the findings. We found increased FC of MTL subregions to Entorhinal cortex (one of the first areas to show tangle pathology in the disease) in prodromal AD patients, but also evidence of the aforementioned, widely reported disconnection between MTL and neocortex.
Co-registration and surgical planning in epilepsy

Objective: Visualizing implanted subdural electrodes in 3D space can greatly aid planning, executing, and validating resection in epilepsy surgery. Co-registration software is available, but cost, complexity, insufficient accuracy or validation limit adoption. We present a fully automated open-source application, based upon a novel method using post-implant CT and post-implant MR images, for accurately visualizing intracranial electrodes in 3D space.
Methods: CT-MR rigid brain co-registration, MR non-rigid registration, and prior-based segmentation were carried out on 7 subjects. Post-implant CT, post-implant MR, and an external labeled atlas were then aligned in the same space. The co-registration algorithm was validated by manually marking identical anatomical landmarks on the post-implant CT and post-implant MR images. Following co-registration, distances between the center of the landmark masks on the post-implant MR and the co-registered CT images were calculated for all subjects. Algorithms were implemented in open source software and translated into a “drag and drop” desktop application for Apple Mac OS X.
Results: Despite post-operative brain deformation, the method was able to automatically align intra-subject multi-modal images and segment cortical subregions so that all electrodes could be visualized on the parcellated brain. Manual marking of anatomical landmarks validated the co-registration algorithm with a mean misalignment distance of 2.87 ± 0.58 mm between the landmarks. Software was easily used by operators without prior image processing experience.
Significance: We demonstrate an easy to use, novel platform for accurately visualizing subdural electrodes in 3D space on a parcellated brain. We rigorously validated this method using quantitative measures. The method is unique because it involves no pre-processing, is fully automated, and freely available worldwide. A desktop application and source code are available for download on the International Epilepsy Electrophysiology Portal (https://www.ieeg.org/) for use and interactive refinement.
Automatic parcellation of neonate brains
In the absence of a neonatal template with cortical subregion labels, it can be extremely difficult to obtain cortical parcellation of new neonatal brain images automatically. We address this problem by utilizing adult templates with rich cortical annotation and a neonatal template with simple tissue labels. Theoretical feasibility is assured because of the preservation of brain putative cytoarchitectonic boundaries from birth to adulthood. We use large deformation registration to propagate neuroanatomical labels from adult to neonatal brain and perform multi-atlas labeling based on accurate prior-based tissue segmentation. We evaluate the repeatability of the labeling by cross-validation with training and testing data.
References:
SES, CHILDHOOD EXPERIENCE, AND THE NEURAL BASES OF LEARNING
This research seeks to understand the relations among socioeconomic status (SES), early life experience, and learning in adolescents. Previous research has shown that learning ability is positively correlated with SES, and preliminary studies suggest that the effects of childhood experience on hippocampal development may in part account for this. The present research will test hypotheses concerning the nature and causes of the SES disparity in learning ability by examining its scope and limits across different types of learning and different neural systems, and assessing its relation to early experience, including stress, stress-buffering parental nurturing and related constructs. The relation of these laboratory-based measures to student learning in school will also be investigated. The methods to be used include (a) behavioral tasks, developed within the multiple memory systems framework of cognitive psychology and cognitive neuroscience, to assess different forms of memory, (b) structural and functional neuroimaging studies of memory systems and (c) prospectively collected data on childhood experience and SES from a longitudinal study of adolescent participants. The relevance of this research to the mission of NIH lies in the crucial role played by learning in the academic, occupational and personal lives of all Americans, and the prospect of preserving and fostering the learning ability in at-risk youth though the application of insights from the cognitive neuroscience of memory, stress, and early experience.
Source: NIH Research Portfolio Online Reporting Tools
Project number: 5R01HD055689-05
PI: Dr. Martha Farah
The International Consortium on Brain MRI of Neonates with Congenital Heart Defects

The International Consortium on CHD Neonatal Brain MRI was established to coordinate efforts to tackle this problem of MR data normalization and provide an open common platform with tools for processing and analyzing CHD brain imaging data in preoperative neonates. This multi-institutional collaboration has obtained the commitment of nine members from Europe, North America, Australia and New Zealand. Consortium members have and will contribute all existing preoperative MR images of CHD neonates acquired as part of study protocols at each institution. The total number of subjects involved will be over 500. The funding for the Consortium came from Saving Tiny Heart Society.
The aims of the Consortium include: (1) developing a set of high resolution structural MRI templates of preoperative CHD brains; (2) constructing a probabilistic injury map with anatomical reference based on manual and automatic segmentations. This paper presents preliminary results from the first attempts to normalize and process the data from the Consortium members and fulfill the first aim. The second aim will be accomplished based on the first aim and the methods to extract white matter lesion that are being developed.
Our strategy to normalize the Consortium data set was to utilize an advanced deformable image registration method to construct a set of templates with optimal shape and appearance. The adopted method was able to transfer individual images into a common template space and tolerate difference in tissue contrast, image resolution and inter-subject variability. A collection of templates were built based on cohorts with a unique characteristic, such as site, age and diagnosis. Subsequent volumetric analyses were performed to measure the tissue and regional brain volumes. Visual inspection confirmed that the constructed templates were of sufficient quality. They aided in normalizing subject images and subsequent segmentation of tissues and lobar regions. Results on data from different sites showed consistent distributions and brain growth in two CHD subtypes did not differ significantly.
Overview of the Tracking the Outcome of Toddlers (TOT) study
Children growing up in poverty are at increased risk for poor cognitive outcomes, arguably one of the most critical issues facing our nation today. While many studies have shown that effects of poverty are apparent by age two years, far less is known regarding effects in children younger than two years. When do the effects of poverty first exert their effects? What cognitive systems are affected? Are changes detectable in the developing brain? What environmental factors are most influential?
This study will:
1) provide novel data regarding neural effects of poverty;
2) identify timing of effects and specific cognitive systems affected by poverty;
3) explore relation of neural structure and function with cognitive outcomes; and
4) assess mediation of poverty’s effects by maternal, child, and environmental factors.
Our long-term goals are to improve outcome of disadvantaged children through: 1) identifying the earliest time period at which, and specific systems for which, interventions can be implemented; and 2) designing interventions based on changes in the developing brain and on environmental risks most strongly associated with cognitive outcomes. We propose to accomplish these aims through enrollment of 60 healthy, African American female term gestation infants born into families with low (n=30) or high (n=30) income in a prospective study. Child assessments will be conducted from birth through age 12 months as follows: Neuroimaging: MRI (major white tracts, cortical regions and hippocampus) at 1 and 12 months; and Infant Development: general cognitive development, language, and executive function at 6 and 12 months. Maternal, child, and environmental factors considered mediators of the effects of poverty on child outcome will be assessed starting in the prenatal period and continuing through infant age 12 months. Maternal factors include nutrition, stress, depression, and IQ. Child factors include temperament, growth, and health. Environmental factors include parental education and occupation, home environment, and childcare. For evaluation of neural and cognitive outcomes, cross- sectional and longitudinal analyses will be conducted with income group as the primary independent variable, followed by mediational analysis.
This is the first prospective study that leverages the use of neuroimaging in the study of neural and cognitive outcomes of infants growing up in poverty. The inclusion of neuroimaging provides not only the first assessment of poverty’s effect on the developing brain of infants, but also an unprecedented opportunity to link brain structure and behavior, and in turn explore mediational mechanisms affecting development of very young children. Successful achievement of proposed aims not only will advance scientific knowledge but also be hypothesis generating regarding causality, reversibility, and prevention of injurious effects of poverty.
Source: NIH Research Portfolio Online Reporting Tools
Project number: 3R21HD072461-02S1
PI: Dr. Hallam Hurt
Structure Specific Analysis of white and gray matter in the rat brain
Tract specific Analysis (TSA) uses continuous medial modeling for structure- specific analysis of diffusion in sheet-like white matter tracts in the human brain. The medial geometry provides both a skeleton surface (the medial surface) and two “spoke” vectors from the skeleton to the boundary of the structure. Diffusion properties such as fractional anisotropy (FA) are sampled along the spokes and their mean is projected onto the skeleton for statistical testing on the medial surface. TSA enhances sensitivity and reduces multiple comparisons.
In a new abstract to appear at the Joint Annual Meeting ISMRM-ESMRMB 2014, we construct medial models of white matter tracts in the rat brain. We further apply the medial modeling approach to include the isocortex. Compared to human cortex, the lissencephalic rat cortex is readily modeled with a medial surface and we can resolve higher FA with DTI. We apply the new medial models to the study of the neurobiology of chronic stress in rats. Tracts connecting to the hippocampus and prefrontal cortex are of particular interest, since these areas are widely reported to be affected by stress and are important in mediating stress response. We therefore built medial models of corpus callosum, fimbria / fornix, and the internal capsule.
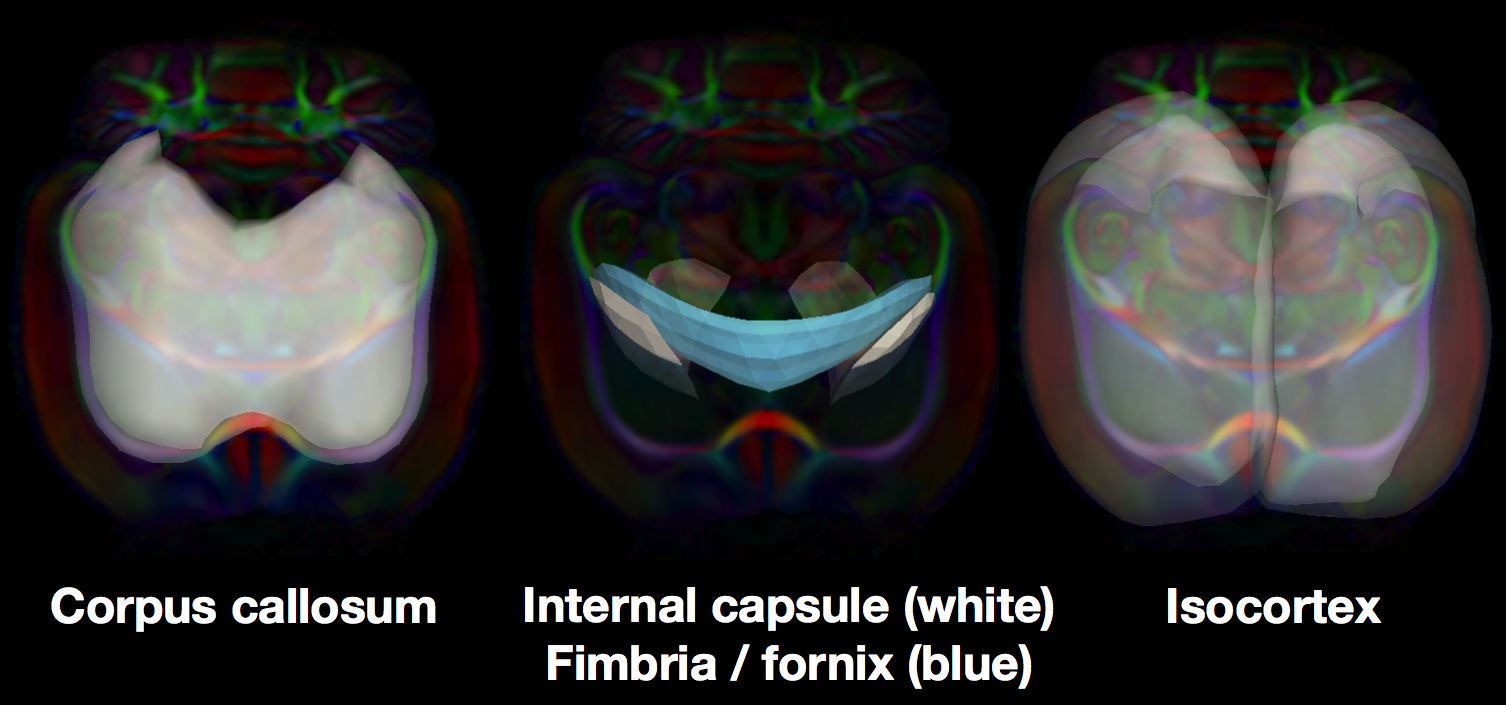
Medial skeletons of structure-specific analysis models in the white brain, overlaid on the RGB-FA image of a rat template.
The results will be presented as an e-poster in Milan.
Beta version of ITK-SNAP 3.0 is released
Version 3.0 is the first major release of ITK-SNAP that is funded by the NIH grant R01 EB014346, “Continued development and maintenance of the ITK-SNAP 3D image segmentation software.” This version includes an almost complete rewrite of the software, along with new features focused on multi-modality image support.

The biggest change in ITK-SNAP is how the tool handles multi-modality images. Even though overlay images were supported in version 2.x, they really shine in ITK-SNAP 3. There are new tools for visualizing and managing overlays, and crucially, you can use multiple modalities for automatic segmentation.
It took two years, but we created a next-generation user interface for ITK-SNAP using the Qt4 library, replacing the outdated FLTK library, and ensuring future viability of the tool. The new GUI provides greatly improved interactivity and added functionality. It takes fewer mouse clicks to accomplish common actions, making ITK-SNAP easier to use and learn. The code behind the new GUI is much better structured, which will make it easier to add new functionality in the future.
Fusing functional signals by sparse canonical correlation analysis improves network reproducibility
We contribute a novel multivariate strategy for computing the structure of functional networks in the brain from arterial spin labeling (ASL) MRI. Our method fuses and correlates multiple functional signals by employing an interpretable dimensionality reduction method, sparse canonical correlation analysis (SCCA). There are two key aspects of this contribution. First, we show how SCCA may be used to compute a multivariate correlation between different regions of interest (ROI). In contrast to averaging the signal over the ROI, this approach exploits the full information within the ROI. Second, we show how SCCA may simultaneously exploit both the ASL-BOLD and ASL-based cerebral blood flow (CBF) time series to produce network measurements. Our approach to fusing multiple time signals in network studies improves reproducibility over standard approaches while retaining the interpretability afforded by the classic ROI region-averaging methods. We show experimentally in test-retest data that our sparse CCA method extracts biologically plausible and stable functional network structures from ASL. We compare the ROI approach to the CCA approach while using CBF measurements alone. We then compare these results to the joint BOLD-CBF networks in a reproducibility study and in a study of functional network structure in traumatic brain injury (TBI). Our results show that the SCCA approach provides significantly more reproducible results compared to region-averaging, and in TBI the SCCA approach reveals connectivity differences not seen with the region averaging approach.
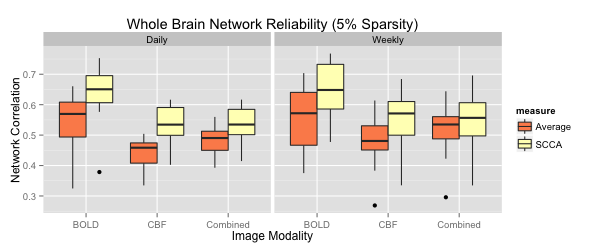
For each metric, using both region averaging (orange) and SCCA (yellow), connectivity matrices were calculated from ASL data acquired in separate acquisitions in the same day and for data acquired one week apart. Whole network correlations were then calculated to examine reliability for the daily (left) and weekly (right) data for each subject. Here we illustrate results using sparsity values of s=t=0.05. A range of sparsity values (s=t) up to 0.25 were examined and these higher values did not produce qualitatively different results.